The pharmaceutical industry is increasingly data-driven, with big data playing a crucial role in the development, manufacturing, and distribution of drugs. However, the sheer volume, variety, and velocity of data generated across the pharmaceutical manufacturing landscape pose significant challenges. From the integration of disparate data sources to the adoption of advanced analytics, navigating these big data challenges is essential for companies aiming to optimize production, ensure regulatory compliance, and ultimately improve patient outcomes.
The Importance of Big Data in Pharma
Big data in pharmaceutical manufacturing encompasses a wide array of data sources, including clinical trial data, patient records, supply chain information, and real-time manufacturing data. The ability to analyze and leverage this data is crucial for making informed decisions that drive efficiency and innovation. For instance, predictive analytics can help forecast demand, optimize production schedules, and reduce waste, while real-time monitoring can ensure consistent product quality and compliance with regulatory standards.
However, the potential of big data in pharma is often hindered by several challenges, ranging from data integration issues to the need for specialized expertise.
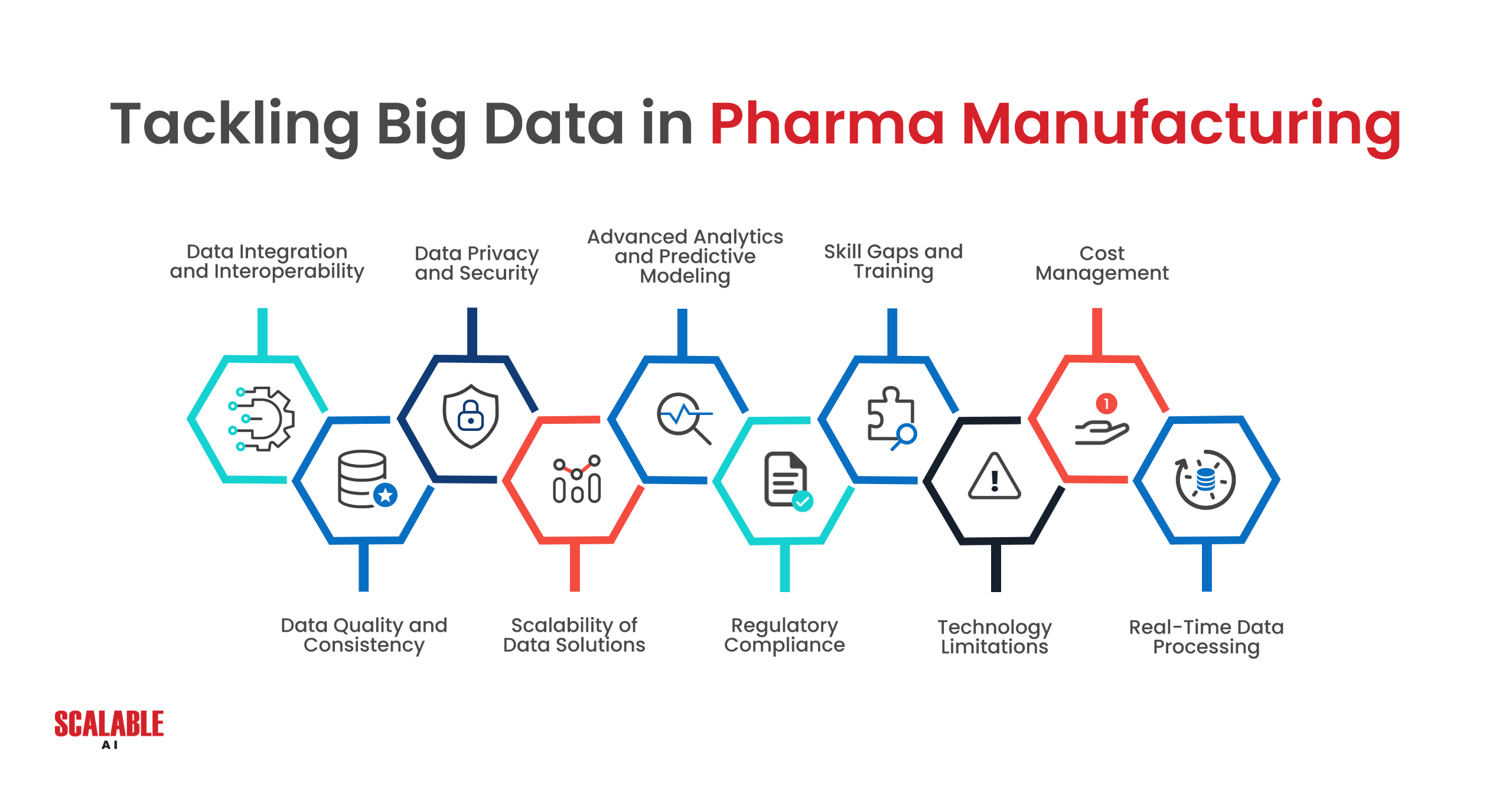
Data Integration and Interoperability Challenges
One of the most significant challenges in pharmaceutical manufacturing is integrating data from various sources. Data is often siloed within different departments or systems, making it difficult to obtain a unified view of operations. For example, clinical trial data might be stored separately from manufacturing data, and integrating these datasets requires robust data management solutions.
Moreover, the lack of interoperability between systems further complicates data integration. Different systems may use different data formats or standards, making it challenging to combine data in a meaningful way. This fragmentation can lead to inefficiencies and hinder the ability to make data-driven decisions across the manufacturing process.
Quality and Consistency of Data
The quality and consistency of data are critical for effective analysis. In pharmaceutical manufacturing, data must be accurate, complete, and timely to ensure that insights derived from it are reliable. However, data is often messy, with inconsistencies or gaps that can skew analysis. For instance, variations in how data is recorded across different sites or departments can lead to discrepancies that are difficult to reconcile.
Addressing these issues requires implementing data governance practices that standardize data collection and ensure data integrity. This might involve adopting standardized data formats, implementing rigorous data validation processes, and providing training to staff on best practices for data entry and management.
Lack of Specialized Expertise
Another significant challenge is the shortage of specialized expertise required to manage and analyze big data effectively. Data scientists and analysts who are proficient in handling large datasets and extracting actionable insights are in high demand, yet they are often scarce within the pharmaceutical industry.
Pharmaceutical companies need to invest in building in-house data science teams or partner with external experts to bridge this gap. Additionally, fostering a culture of data literacy across the organization can empower more employees to engage with data and contribute to data-driven decision-making.
Transitioning from Traditional to Advanced Analytics
Many pharmaceutical companies still rely on traditional data management solutions that are not equipped to handle the complexities of big data. These legacy systems often lack the flexibility and scalability needed to process and analyze large volumes of data efficiently. Transitioning to advanced analytics platforms that support real-time data processing and predictive modeling is essential for staying competitive in today’s data-driven landscape.
However, this transition can be challenging, as it often requires significant investment in new technology and infrastructure. Additionally, companies must ensure that their data management practices evolve in tandem with their technology to fully realize the benefits of advanced analytics.
Ensuring Data Security and Compliance
Data security and compliance are paramount in the pharmaceutical industry, where the handling of sensitive patient data and proprietary information is subject to strict regulations. Ensuring that data is secure and that privacy is maintained throughout the data lifecycle is a significant challenge, particularly when dealing with large, distributed datasets.
Companies must implement robust cybersecurity measures and adhere to regulatory requirements, such as the GDPR in Europe or the HIPAA in the United States. This involves not only securing the data itself but also ensuring that data access is controlled and monitored, and that employees are trained on best practices for data security.
The Future of Big Data in Pharmaceutical Manufacturing
Despite the challenges, the potential benefits of big data in pharmaceutical manufacturing are immense. As companies continue to invest in advanced analytics and data management solutions, they will be better equipped to optimize their manufacturing processes, improve product quality, and accelerate the development of new drugs.
Moreover, as the industry moves towards greater digitization and automation, the ability to harness big data will become increasingly critical. Companies that successfully navigate these challenges and leverage big data to its full potential will be well-positioned to lead the way in pharmaceutical innovation.
Conclusion
Navigating the challenges of big data in pharmaceutical manufacturing is no small feat, but it is essential for companies looking to stay competitive in an increasingly data-driven industry. By addressing issues related to data integration, quality, expertise, and security, pharmaceutical manufacturers can unlock the full potential of big data, driving innovation and improving patient outcomes. As the industry continues to evolve, those who embrace big data and invest in the necessary tools and expertise will be at the forefront of pharmaceutical progress.
Read Whitepaper Data Powerhouse: Leveraging Big Data in Life Sciences
